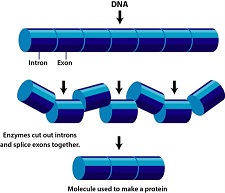
 Biochemistry III now provides you with a more in depth look at the biochemical processes that drive all animal life on Earth.
Biochemistry III now provides you with a more in depth look at the biochemical processes that drive all animal life on Earth.
This course will build on your existing knowledge to help prepare you for work in a human or animal health or science related profession, as practitioner, educator or researcher. This course extends from enzymes, glycolysis, fatty acid oxidation to electron transport and lipid and animo acid metabolism and more.
Prerequisite: Biochemistry II or equivalent